Here is a compilation of notes on Odour. After reading these notes you will learn about: 1. Meaning of Odour 2. Sources of Odour 3. Hypothesis 4. Effect on Human Health 5. Measurement and Monitoring.
Contents:
- Notes on the Meaning of Odour
- Notes on the Sources of Odour
- Notes on the Hypothesis of Odour
- Notes on the Effect of Odour on Human Health
- Notes on the Measurement and Monitoring of Odour
Note # 1. Meaning of Odour:
Odour can be defined as the “perception of smell” or in scientific terms as “a sensation resulting from the reception of stimulus by the olfactory sensory system”. Whether pleasant or unpleasant, odours are induced by inhaling air-borne volatile organic or inorganic substances.
The smell is one which causes discomfort and distress but odour is one which under different conditions can be considered pleasing. Odourous gases emitted from a source are carried & spread away by wind movement in atmosphere. In fact, the osmogenic molecules (the smell) enter the nasal cavity through the nostrils and excite the osmoceptors.
The specific message travels in the form of specific electric discharges along the myelinated nerve fibres to the receptor centres in the brain. After processing the message it is delivered to the evaluation centres in the brain where olfactory information is compared with stored experience (the memory).
The interpretation of message is then relayed back along other nerve paths. In fact, there is a nervous mechanism in brain acting like a rectifier.
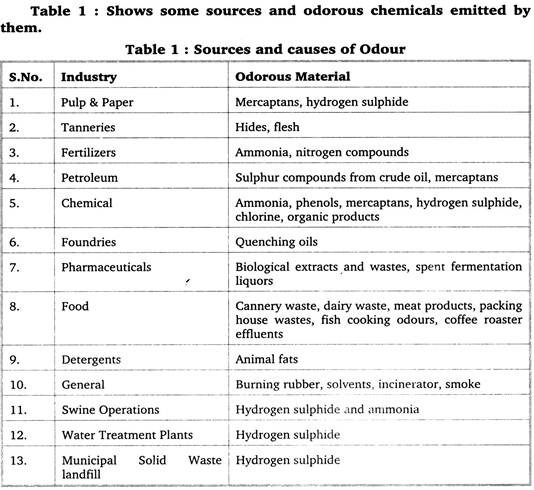
Note # 2. Sources of Odour:
Most commonly reported odour-producing compounds are hydrogen sulphide (having rotten egg odour) and ammonia (with sharp pungent odour). Carbon disulphide, mercaptans, product of decomposition of proteins (especially of animal origin) phenols and some petroleum hydrocarbons are other common odorants.
Most offensive odour is created by the anaerobic decay of wet organic matter such as flesh, manure, fodder or silage. Odour originating from livestock manure is the result of 168 odour-producing compounds. Warm temperatures enhance anaerobic decay and foul odour production.
Odour can arise from many sources. Most of the sources are man-made e.g. Garbage. Improper dumping on vacant land is a common phenomenon. It leads to foul smell due to putrefaction of dumped garbage. Unscientific design of landfill, increased sewage production & improper sewage treatment practices produce unpleasant odour.
Large livestock operations, poultry farms, tanneries, slaughterhouses, food and meat processing industries and bone mills are among major contributors to odour pollution. Agricultural activities like decaying of vegetation, production and application of compost etc. also contribute to odour pollution.
Odour sources can be four types:
i. Point Sources:
Point sources are confined to emissions from vents, stacks, (e.g. garbage) and exhausts with a known flow rate.
ii. Area Sources:
Area sources may be unconfined like swine operations, sewage treatment plants, waste water treatment plants, solid waste landfills, composting, household manure spreading, settling lagoons or a cattle feedlot etc.
iii. Building Sources:
Building sources of odour may be like those from hog confinement, chickens and pig sheds.
iv. Fugitive Sources:
Source of this type of odour emission include bed or bio-filter surface. The emission normally has an outgoing or upward gas flow of garbage.
Improper handling of public amenities like toilets of cinema halls bus/railway stations, hospitals, shopping complexes etc. generate pungent odour, which affects the users as well as neighbourhood residents.
Note # 3. Hypothesis of Odour:
Today there are two hypotheses of odour-the one maintaining that chemical reactions are the cause of olfactory stimulation and the other pointing out that it is the physical causes which set the olfactory nervous process into operation. In each group, several variants have been proposed.
The conception that osmogenic stimulation is chemical in nature is the older of the two. Moncrieff has reviewed most chemical hypotheses and reveals many interesting details of this aspect of olfaction. During the last twenty years. Amoore has proposed a new approach by assuming the olfactory stimulus to be essentially a matter of molecular shape.
One of the earliest mentions of molecular morphology as the fundamental datum of odour discrimination is found in a paper by Jones and Pyman. They assumed that the shape of the whole molecule rather than the structural form of its side chains was the decisive factor.
Emial Fisher (1852-1919), a German biochemist, proposed the lock and key in interaction of molecules and Paul Ehrlich (1854-1915), also a biochemist, developed, from Fisher’s principles, the side-chain theory named after him.
The scientist Moncrieff (ibid) confirms the shape hypothesis by writing in 1949, “it seems likely that to be odorous, a substance must have molecules of prescribed shapes which will fit on certain available molecular sites in the olfactory receptors.”
According to Amoore (1952), it is assumed that the hyperline organization of the ultimate odour elements in the olfactory epthelium has a surface structure which is best described as pockmarked or pitted. The shapes of these depressions are not haphazard, but show certain forms of a defined regularity.
Those molecules, whose overall configurations happen to be identical with or similar to the shape of certain of these pits, will fit wholly, or partly, into the appropriate depression in the epithelium. This is called as ‘lock and key’ organization and results in the molecules being so close to the osmoceptors that a sensation of odour will be produced.
The main character of the odour is determined-one must assume as a logical conclusion-not by the shape of the molecule but by the fact that some chemically quite unrelated substances may evoke identical, or very similar, odours- for instance Camphor C10H16O; Silicononyl alcohol Si(C2H5)3. C2H4OH and Durene C6H2(CH3)4.
Another camphoraceous combination is Hexachloroethane C6Cl6 and Trinitroacetonitrile (NO2)3CCH.
Amoore has allocated definite shapes to molecules of definite odours thus:
(1) Camphoraceous: Sphere
(2) Musky: Disc
(3) Floral Diamond
(4) Pepperminty: Wedge.
The physical hypothesis is based on the fact that molecules of matter are in continuous motion which has different modes.
They are known to the physicist as rotational oscillations and vibrational oscillations, both producing radiations, the wavelengths of which are characteristic of, and different for each mode and each molecule. Values of these wavelengths have been calculated and are in conformity with experimental results.
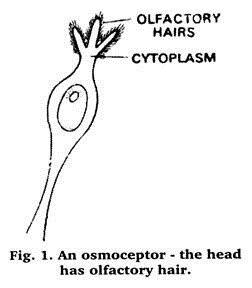
One of the most significant discoveries in biology in recent years demonstrated odour-sensitive elements which at one time were thought to be the ultimate structure, yet displayed a hyperfine organization of the minutest cavities from the very heart of which a hair emerged reaching just above the surface of that cavity (Figure 1).
To the physicist this structure looks like a microminiaturized magnetron (an electronic valve number, having a cavity for resonating operation in microwave range).
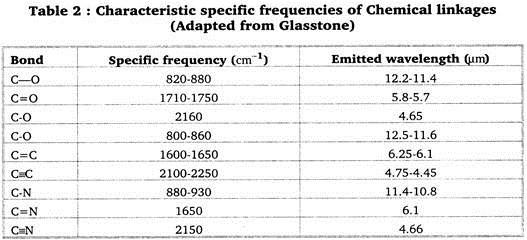
This is shown in Table 2.
It is noticed that if one atom in a chemical link is much heavier than the carbon atom the frequency of radiation is reduced, i. e., the wavelength increases. It is, one might imagine, more difficult for the large atom for instance chlorine (CI…35.5) to follow the vibrations of the carbon atom (C…12). The specific frequency of the C—CI bond is only 650—710 cm-1, and the wavelength correspondingly 15.4—14.1 µm.
It has been found that radiation of wavelength λ1 striking a molecule will be scattered and will suffer a change in wavelength to λs. The difference between incident wavelength λ1 and scattered wavelength λs is called Raman shift and is independent of the absolute value of both waves, thus
1/Δλ = 1/λi -1/λS
and, substituting wavenumbers ![]() = 1/λ the Raman shift can be written as Δ
= 1/λ the Raman shift can be written as Δ![]() = – vs.
= – vs.
The experimental results have shown that osmogenic substances have characteristic Raman shifts which fit well into the range 1500 < Δv < 3500 so that the Raman spectrum extends from about 7µm to 3µm or less. Some typical Raman shifts are: aldehydes 1700, acetylenes 2100, sulphydryls 2500, and aromatic hydrocarbons 3000.
Dyson noticed that Raman shifts below 1500 and beyond 3000 are indicative of odourless substances. These findings are of importance when considered together with anatomical data about the fine structure of osmoceptors.
As the infra-red wavelengths of ‘odour radiation’ are in the range of from 1-15 µm it is clear that any anatomical structures acting as physical elements in the process of transmission must be.
Note # 4. Effects of Odour on Human Health:
Odour affects human beings in a number of ways. Strong, unpleasant or offensive smells can interfere with a person’s enjoyment of life especially if they are frequent and/or persistent.
Major factors relevant to perceived odour nuisance are:
i. Offensiveness
ii. Duration of exposure to odour
iii. Frequency of odour and
iv. Tolerance of the receiver
Toxic stimulants of odour may cause ill health damage the respiratory symptoms. Secondary effects, in some, may be nausea, insomina and discomfort. Very strong odour can result in nasal irritation; trigger symptoms in individuals with breathing problems or asthma.
Soot and dust, when inhaled, certainly affect the respiratory organs. Normal air contains dust but this is quickly removed once it has reached deeper structures of the lungs. The sticky muscus on the nasal mucosae holds back coarser dust particles thus preventing them from entering the remoter respiratory passages. The trachea and bronchial tubes are also coated with muscus reinforcing the cleaning effect.
Mouth breathing cannot make use of the cleaning action afforded by the nasal mucosae and loses this very important line of defence. The mucociliary activity of respiratory epithelia is one of the basic functions that the respiratory organism relies upon in responding to unfavourable environments, such as air pollutants, especially of the solid particulate type.
However finest dust particles will reach the deepest structures of the lung. This would, in time, become a threat to life.
The major factor controlling the distribution of inhaled gases and particles in the alveolar air is molecular diffusion. Not more than about 15% of the inspired air is mixed mechanically during breathing. It has also been proved that air pollution affects the respiratory system.
At the age of 40, black dust, silica, number of dust foci of 1 mm diameter are deposited in lungs of people. Liquid particulate matter and radioactive iodine also reaches the lungs.
It has been found that in the vicinity of industrial units, there is danger of hot particles entering the lungs. Autoradiographic analysis has revealed the presence of hot particles which show a regular inhibition of mitosis (division of active somatic cells and germs cells) in the host tissue.
A respiratory tract dummy has been designed which serves as a model of the tract, in particular as a retention simulator. A four-stage filter simulates the human respiratory tract. The above data apply not only to solid particulate matter, but also to osmogenic pollutants in gaseous form.
Whilst gross mechanical pollution of the air by soot, grit and smoke has been given the medical certificate of ‘highly dangerous’ and noise is also labelled as ‘undesirable, even detrimental, to people irretrievably caught up in the bedlam of industrial production, medical opinion views air pollution by smells as nothing worse than a molestation, unless the ingredients are toxic.
Generally, osmogenic fumes are non-toxic; the popular fallacy is sometimes maintained that a person’s health has been ruined by that smell. In lawsuits concerning osmogenic air pollution the minds of all involved are difficult to screen from preconceived ideas.
Researches have shown that a few gaseous compounds exist which may present real hazards to man. The main danger lies not in their odour or in their toxicity alone but in the aggravation of circumstances stemming from the fact that the maximum admissible concentration is lower than the odour threshold so that not even the nose, this most sensitive detector of smell, can perceive the danger in time.
If the ratio of olfactory threshold MAC is less than unity, the smell will be perceived by the nose before the pathogenic concentration is reached. For unity ratio the slightest impairment in olfactory function will raise the threshold value above MAC level and ratios greater than unity indicates danger when relying on the nose instead of using an instrumental detector.
The cases threshold MAC < 1 and threshold MAC > 1 are clear cut. No danger in the first, immediate danger in the later instance. Only when the ratio equals or approaches unity there is hidden danger. Ozone is an instance. Several years ago, its maximum acceptable level was put at 1 ppm. by some at 0.8 ppm.
Russian publications strongly suggested a reduction to 0.5 ppm and finally, the American Conference of Governmental Industrial Hygienists standarized it at 0.1 ppm. German hygienists now accept only one half that values 0.05 ppm. Nasr has reviewed the biochemical aspects of ozone intoxication.
Note # 5. Measurement and Monitoring of Odour:
Odours are measured adopting olfactometric testing methods, which are psycho-physical methods. In these methods, the olfactory responses of individuals sniffing diluted odour presented by an olfactometer to determine odour strength or odour concentration.
Sampling of Odours:
Odour measurement requires representative samples of the air to be drawn into a sample bag and rapidly transported to an odour laboratory for olfactometric testing. Sampling strategies and techniques depends on emission sources characteristics. Each type of source has special requirements for sampling and sample collection.
Point sources:
Odour samples are taken into Tedlar sampling bags loaded in a vacuum drum through Teflon tube inserted into the stack at different points.
Area sources:
A portable wind tunnel system can be used to determine the specific emission odour rate (SEOR). The quantity of odour is calculated from the concentration of odour (as measured by olfactometry), which is then multiplied by the volume of air passing through the hood per unit time.
Building sources:
From animal sheds odour samples are normally taken from several points within a shed.
Fugitive sources:
Odorants in the atmosphere or gas stream can be collected by passing known volume of air or gas through a column of activated carbon or by condensing techniques.
Measurement of Odour:
The odour measurement by alfactometric methods can be divided into two parts:
(a) Determination of the threshold concentration of odoriferous gases:
For determining threshold concentration by the olfactometery testing procedure, a diluted odorous mixture and an odour free gas (as a reference) are presented separately from two shiffing ports at 20 1pm to a group of eight panelists in succession.
In comparing the gases emitted from each port, the panelists are asked to report the presence of odour. The gas dilution ratio is then decreased by a factor two. The panelists are asked to repeat their judgement.
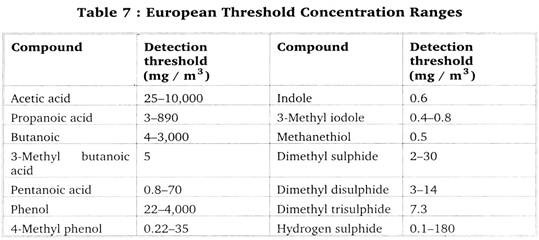
This continues for six different dilution levels, resulting a total of 8 × 6 × 2 = 96 judgments (sniffing) from eight panelists. Using panelist responses odour concentration expressed as odour unit per cubic meter (mg/m3) can be calculated from individual threshold estimates. Threshold concentration ranges for some unpleasant odours are presented in Table 7.
(b) Determination of the type and intensity of odour:
For odour intensity measurement, generally a panel of 6 to 12 persons of normal health is employed. The panel members sniff the air at a given location at the same time and report individually the nature and intensity of the odour. By averaging the values recorded by members of the panel, a single value can be assigned to the odour intensity at a given location.
Generally odour intensity increases with the odorant concentration. The relationship between intensity and concentration can be expressed as:
P = K log S
where P = Odour intensity
K = Constant
S = Odour Concentration
The preferred and internationally standardized methods of measuring odours are the Dutch Standard Method (NVN 2820) and the more recent European Method (CEN TC 264). A joint Australia New Zealand standard based on the draft CEN standard is in the process of preparation.