Here is a compilation of notes on Atomic Absorption Spectroscopy. After reading these notes you will learn about :-1. Meaning of Atomic Absorption Spectroscopy 2. Principle of Atomic Absorption Spectroscopy 3. Advantages 4. Disadvantages 5. Instruments 6. Experimental Techniques 7. Interference 8. Applications.
Contents:
- Notes on the Meaning of Atomic Absorption Spectroscopy
- Notes on the Principle of Atomic Absorption Spectroscopy
- Notes on the Advantages of Atomic Absorption Spectroscopy
- Notes on the Disadvantages of Atomic Absorption Spectroscopy
- Notes on the Instruments of Atomic Absorption Spectroscopy
- Notes on the Experimental Techniques of Atomic Absorption Spectroscopy
- Notes on the Interference of Atomic Absorption Spectroscopy
- Notes on the Applications of Atomic Absorption Spectroscopy
Note # 1. Meaning of Atomic Absorption Spectroscopy:
Atomic Absorption Spectroscopy was invented by Alan Walsh in 1950’s for the qualitative determination of trace metals in liquids. The superiority of the technique over other is based on the fact that by this technique 50-60 elements can be determined without any interference from trace to big quantities.
All these elements can be detected here which fail to yield satisfactory result in flame photometry. Thus, it is a successful instruments for detection and estimation of metals and non-metals both types of pollution from factories.
The technique has also proved very helpful to both aqueous and non-aqueous solutions.
Note # 2. Principle of Atomic Absorption Spectroscopy:
When a solution having a mixture of metallic species is introduced into the flame, the solvent evaporates and vapour of metallic species is obtained. Some of metal atoms can be raised to an energy level sufficiently high to emit characteristics radiation of metal-a phenomenon that is used in flame photometry. Here a large amount of metal atoms remain in non-emitting ground state.
These ground state atoms of a particular element are receptive of light radiation of their own specific resonance wavelength. In this way, when a light of this wavelength passes through a flame, a part of light will be absorbed and this absorption will be proportional to the intensity of atoms in the flame.
So in atomic absorption spectroscopy the amount of light absorbed is determined because the absorption is proportional to the concentration of the element.
Note # 3. Advantages of Atomic Absorption over Flame Photometry:
(1) It does not suffer from spectral interference, which occurs in flame emission spectroscopy.
(2) It is independent of flame temperature.
(3) By atomic absorption technique, traces of one element can easily be determined in presence of high concentration of other elements.
(4) It has proved very successful in the analysis of bronze and copper alloys and in the determination of metals like platinum, gold etc.
Note # 4. Disadvantages of Atomic Absorption Spectroscopy:
Some of the disadvantages are summarized as follows:
(1) This technique has not proved very successful for the estimation of elements like V, Si, Mo, Ti and A1 because these elements give oxides in the flame.
(2) In aqueous solution, the anion affects the signal to a noticeable degree.
(3) A separate lamp is needed for the determination of each element. Attempts are being made to overcome this difficulty by using a continuous source.
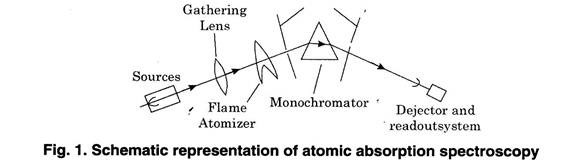
Note # 5. Instruments of Atomic Absorption Spectroscopy:
The apparatus consist of:
(1) Radiant Source.
(2) Atomizer
(3) Monochromator
(4) Lenses and Slits and
(5) Detectors.
The main components used in the instrument can be described as follows:
(1) Radiant Sources:
Generally a hydrogen lamp is used as continuous source of radiation.
(2) Atomizer:
Generally burners are used to break the liquid sample into droplets which are then allowed to enter into flame. The droplets are then evaporated and sample element is left in residue. The residue is then decomposed by flame. Thus in this process the sample is reduced to atoms.
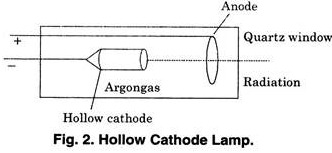
(3) Hollow Cathode Lamp:
For atomic absorption spectroscopy the radiation source is a hollow cathode lamp (shown in figure 2).
It consists of the following parts:
(i) Cathode: is made of the element to be determined or coated with it.
(ii) Anode Anode is made of tungsten, zinconium or nickel.
(iii) Window is made of Pyrex glass depending on wavelength of emitted radiation.
(iv) The lamp is filled with neon or argon gas.
These gases emit sharp line spectra.
Generally these lamps are constructed for individual elements but multi-element lamps have also been prepared for all purposes.
The hydrogen lamp is a hollow cathode lamp.
A hollow cathode lamp emits more than one composite line for each element but the required spectral line can be separated by means of a relatively low dispersion monohromator. Most of lines are non-absorbing lines because they involve transition other than from ground state. The most intense absorption line is selected to provide maximum intensity.
The inlet and exit slit widths of monochromator should be narrow to isolate the particular line being used; the requirements depend on:
(1) Focal length,
(2) Grating ruling of Monochromator.
(4) Monochromator:
Generally the monocchromators are gratings and prisms.
(5) Filters or slits:
Filters or slits are used for isolation of required spectral line if element has a simple line spectrum. Filter photometers are used for determination of potassium, sodium calcium, magnesium etc. in samples.
(6) Detectors:
Generally photomultipliers are used as detectors. In some instruments two filters and two detectors are used to compensate the fluctuation in the sources.
Note # 6. Experimental Techniques of Atomic Absorption Spectroscopy:
First of all, a meter is adjusted to read zero absorbance or 100% transmittance when a blank solution is sprayed into the flame and light of hollow cathode lamp passes on to photomultiplier tube. Now the solution to be investigated is introduced, a certain part of light is absorbed resulting in decrease of light intensity falling on photomultiplier. This gives a deflection in the meter needle which is noted immediately.
As this is a comparative method hence standard solutions of elements are used to make a calibration curve from which the concentration of sample elements can be calculated.
Note # 7. Interference of Atomic Absorption Spectroscopy:
(1) Chemical Interference:
In practice, it has been found that phosphate ions interfere with determination of calcium and magnesium. This interference can be reduced by adding a salt of lanthanum.
(2) Ionisation Interference:
Ionisation interference is caused due to alkali metals which need low energy for ionization. For example, a loss in petrochemicals sensitivity results due to splitting of a free metal atom into a positive ion and an electron
M = M++ e-
(3) Role of Solvent:
Solvent plays an important part to interfere in the determination of conc. of metals. The results have shown that metals in aqueous solution yield lower absorbance readings than same concentration of such metals when present in the organic solvent.
(4) Dissociation of Metal Compounds:
Some metals like Al, Ti etc. when subjected to flame give oxides in place of metal atoms and thus complicate the system.
(5) Spectral Interferences:
Sometimes interference occurs due to overlapping of any radiation with that of characteristic radiation of sample element, e.g. potassium doublet (4044, 2047Å), manganese triplet (4031, 4033 and 4035Å).
This interference can be removed by working with AC amplifiers in the technique.
Note # 8. Applications of Atomic Absorption Spectroscopy:
(i) Quantitative Analysis:
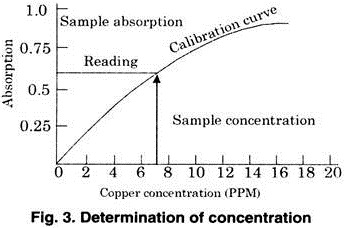
As we know that each element has its own characteristic emission spectrum, hence the intensity of the lines is compared with standard and the concentration can be easily evaluated from the graph (Fig. 3).
Suppose the intensity of unknown element is C, then the concentration is evaluated by drawing a perpendicular on the line (calibration curve) and from the point it cuts the curve. A perpendicular is drawn on the x-axis. The value from (0 to 0) will give the concentration of unknown in moles per litre.
In atomic adsorption spectroscopy, the same method is followed for determining the concentration of the element in an unknown solution.
(ii) Method of Standard Addition:
If calibration graph is linear, the sample concentration can be calculated by adding known amount of the test element to the sample. This gives a section of calibration graph above the unknown sample concentration and the resulting straight line can be extrapolated back to zero signal intensity.
The concentration scale is determined by standard additions and unknown concentration is given by the point at which extrapolated line crosses concentration axis.
(iii) Quantitative Analysis:
Generally first a curve is plotted between absorbance valve vs. concentration of standard samples of the element. A linear curve is obtained.
From this curve, the concentration of unknown is evaluated by knowing absorbance value only from the following equation:
A = S × C
or Absorbance = Slope × Concentration
As it is very sensitive technique hence it gives more accurate results than many analytical methods.