This article throws light upon the top nine treatment processes used for removal of various impurities from water. Some of the treatment processes are: 1. Removal of Suspended & Dissolved Impurities 2. Removal of Dissolved Gases 3. Removal of Gas from Water 4. Removal of Iron and Manganese 5. Removal of Silica 6. Removal of Oil 7. Removal of Slime and Algae from Water and Others.
Treatment Process # 1. Removal of Suspended & Dissolved Impurities:
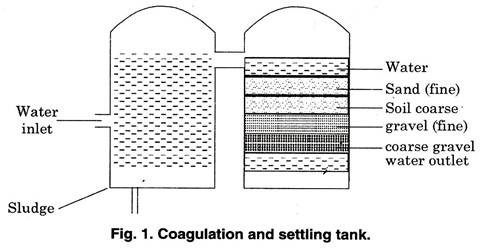
Removal of coarse, dispersed and colloidal impurities from water is known as clarification. These impurities may be removed either by filtration or by sedimentation.
(a) Sedimentation:
The simplest way to clarify muddy water is to allow the suspended material in it to settle, the process is known as sedimentation. In filtration the water is clarified by passing it through a porous material, which retains coarse impurities on its surface and in its pores.
(b) Filtration:
The apparatus used for filtration is called filter and the porous material that fills the filter is known as filtering material or medium.
The process of sedimentation allows solid particles to settle down on the bottom of the settling tank in which water undergoing clarification is at rest or in slow horizontal or upward notion.
(c) Coagulation:
Silt particles are small enough to remain in colloidal suspension that cannot removed by sedimentation. The process of coagulation is used to remove colloidal particles from water.
The process of consolidation of colloidal particles, terminating in precipitation of the substances removed from the treated water by settling or filtration is called coagulation.
The reverse process of coagulation i.e. conversion of a solid into a colloidal state, is called peptization. Reagents that are able to provoke coagulation of natural colloids in water are coagulants. Few examples of coagulants are ferrous sulphate (FeSO4.7H2O), aluminium sulphate [Al2(SO4)3.18H2O] and ferric chloride (FeCl3).
(d) Flocculation:
The process of coagulation could be improved by adding special reagents known as flocculants. Flocculants are the particles absorbed on the dispersed particles and on coagulants flakes to convert them into particles of reasonable large size and high stability. Starch, sodium polyalginate, polyacrylamine etc. are examples of flocculants.
Treatment Process # 2. Removal of Dissolved Gases:
It has been found that some of the gases if present in water in dissolved form may cause certain difficulties. Dissolved carbon dioxide corrodes the pipes. Similarly oxygen, chlorine, and other gases, if in dissolved form, are present in excess amounts, also cause difficulties.
Many dissolved gases can be removed by boiling, decompression or by means of chemical treatment. Except oxygen and nitrogen all other gases can be reduced by aeration. Aeration process removes carbon dioxide, hydrogen sulphide, and odour very rapidly.
Following are some of the methods of aeration:
(i) By mechanically agitating water.
(ii) By diffusing compressed air inside the water.
(iii) Mixing air in water under pressure.
(iv) By spraying water into the atmosphere through nozzles 1 to 2-3 metre.
(v) Flowing water through perforated trays and coke beds, so that the water filters through them.
(vi) By flowing water over weirs, steps etc., so that water is exposed to sun as much as possible.
The presence of iron and copper oxides in water along with oxygen complicates the way in which hydrazine reacts with these substances.
The reaction:
(1) can proceed at higher rate under certain conditions than reaction
(2) Hence reaction
(3) becomes of secondary importance.
Hydrazine can be used to control nitrite corrosion in high pressure boiler units. It is introduced into a boiler unit as a solution of definite concentration by means of metering pumps. It should he noted that hydrazine solutions are noxious and so must be handled with great care.
Under certain conditions excess hydrazine also decomposes according to the reaction.
3N2H4 → 4NH3 + N2
The rate of this reaction depends upon:
(1) The ambient temperature,
(2) pH value of water.
While using hydrazine in heat cycle, it should be noted that accepted or admissible excess amount of hydrazine be decomposed completely in the circuit. It should not be allowed to pass to steam and water consumers.
Treatment Process # 3. Removal of Gas from Water:
Dissolved gases, such as O2, Cl2, CO2 and H2S etc., are removed from water by various chemical and physico-chemical methods.
The chemical treatment of water involves adding special substances to water, which qualitatively react with the dissolved gases. For example, SO2, sodium thiosulphate (Na2S2O, 5H2O) sodium sulphate (Na2SO4) ferrous sulphate (FeSO4) ammonia (NH3) etc., are used to remove chlorine from water. Oxygen is removed from water by iron chips, sulphites, SO2 etc. and CO2 bound with NaOH, Na2CO3, CaO and CaCO3.
(1) 2H2O + Cl2 + SO2 → 2HCl + H2SO4
H2O + Cl2 + Na2SO3 → Na2SO4 + 2HCl
(2) 2Na2S2O3 + Cl2 → Na2S4O6 + 2NaCl
SO2 + 4Fe → 2Fe2O3
The last reaction is especially energetic at high temperatures. At 25°C it continues for 20-30 minutes while at 80°C it gets completed in 3 to 5 minutes.
2Na2SO3 + O2 → 2Na2SO4
H2S can be bound with chlorine with subsequent coagulation of sulphur.
H2S + Cl2 → 2HCl + S
CO2 as carbonic acid (H2CO3) reacts with the chemical agents according to the following reactions.
(1) H2CO3 + NaOH → NaHCO3 – H2O
2H2CO3 + CuO → Ca(HCO3)2 + H2O
(2) H2CO3 + CaCO3 Ca(HCO3)2
Any substance that reacts with gases can be used to remove them from water. They only requirement is the harmlessness of the substances themselves and of the products of their combination with the gases, and also the cost.
The dose of the chemical is determined empirically by trial treatment of water sample, or by stoichiometric calculation provided the concentration of gas contained in the water is known. For example, SO2 reacts with Cl2.
Treatment Process # 4. Removal of Iron and Manganese:
Manganese and Iron are objectionable generally found together, in raw water. Iron is found in the form of ferrous sulphate and ferrous bicarbonates.
The presence of iron and manganese in excess of 0.3 ppm renders water objectionable due to following reasons:
(i) They cause corrosion to plumbing works.
(ii) They cause objectionable taste and odour.
(iii) They cause trouble in various manufacturing process and make them uneconomical.
(iv) They cause spots on clothes during washing or during their use in textiles.
(v) They may make water reddish due to presence of iron.
Removal of iron and manganese can also be done by any one of the following methods:
(i) By base-exchange process.
(ii) By chlorination and
(iii) By aeration of water.
Iron alone in the absence of organic matter can usually be removed by aeration of any type, followed by sedimentation and filtration. Combination of iron and manganese or iron alone loosely bound to organic matter may require aeration in multiple coke trays (Fig.1) containing coke, gravel or crushed pyrolusite (pyrolusite is a negative manganese dioxide).
It has been revealed that metaphosphates may be used to prevent precipitation of iron or manganese. Their use is generally applicable when iron concentration is less than 1 ppm.
The method of removal of iron from water consists in oxidation of Fe2+ to Fe3+ metal and its precipitation as Fe(OH)3. If iron is present in water as hydrocarbonate, it can be removed by aeration.
This salt is hydrolysed in the following way:
Fe(HCO3)2 + 2H2O → Fe (OH)2 + 2H2CO3
H2CO3 ⇋ H2O – CO2
Ferrous hydroxide is oxidized by atmosphere oxygen to Fe(OH)2 . ++
4Fe (OH)2 + 2H2O + O2 4Fe(OH)3
This method can be used to reduce the iron content up to 0.1 to 0.3 mg/litre.
FeSO4 is removed from water by treating it with lime.
FeSO4 + Ca (OH)2 → Fe(OH)2 -CaSO4
4Fe(OH)2 + 2H2O → 4Fe(OH)3
Iron present in organic and inorganic compounds can be removed by this method.
Another method to remove iron is to pass water through a bed of highly dispersed suspension of chalk and aluminium hydroxide. Iron salts are converted into ferrous carbonate by chalk.
FeSO4 + CaCO3 → FeCO3 + CaSO4
FeCO3 is hydrolysed into ferrous hydroxide
FeCO3 + 2H2O → Fe (OH)2 + H2CO3 (H2O + CO2)
Manganese is removed from water by oxidation of the Mn+2 into Mn+4. Water is treated with lime in the presence of pyrolusite or quartz sand with MnO2 film applied onto its grains.
2MnO2 + 2Ca (OH)2 + O2 → 2Ca MnO4 + 2H2O
Hexavalent manganese oxidises divalent manganese into its tetravalent state.
Treatment Process # 5. Removal of Silica:
The following are the methods which may be used for silica removal:
(i) By using magnesium hydroxide with carbon dioxide, calcium bicarbonate or magnesium bicarbonate which produce magnesium carbonate absorbing silica.
(ii) Apply ferric sulphate and line to develop ferric hydroxide which absorbs silica. Silica may be very objectionable impurity in boilers. Since it forms a tetracious scale. Thus water softened by lime soda process is first subjected to demineralization before using in a high pressure boiler.
Coagulation followed by filtration, prechlorination, removal of taste, odour and colour, super chlorination followed by dechlorination and use of chlorine dioxide is the methods which help in the removal of taste, odour and colour.
The deodouration of water is the process by which foul odours of water are removed. Odour can be due to phenols, hydrogen sulphide, chlorine, soluble salts, pesticides etc. and smacks even when they are present in negligible quantities. For example, DDT can smell in as small a concentration as 0.07 mg/litre.
The method most commonly used to remove odour is passing water through activated charcoal.
In order to improve the taste of water containing phenols. Pre-ammoniation is used. Pre-ammoniation precludes the odour of residual chlorine, and decreases the probability of the subsequent development of bacteria and pipe rusting. But this process does not destroy odours and smacks due to micro-organisms. The process involves treating water with ammonia or ammonium sulphate.
Ozonisation:
Ozonisation is another method to improve the taste of water. It is used to separate water from synthetic surface-active substances, but it is effective against pesticides, which are stable and strong oxidants.
Treatment Process # 6. Removal of Oil:
Oil from water can be removed by a number of procedures such as filtration, settling, coagulation etc. These methods are not required if water is clear. In such cases water is filtered through powdered charcoal together with sand filtration.
The oil can also be removed from water using diatomaceous earth filters if oil is present in boiler field water it is essential to remove it, otherwise heat insulating oil film will form on boiler tubes, which may be dangerous.
It may be removed by absorption by passing the water through containers of excelsior.
1. It develops bad taste.
2. It develops corrosion and incrustations in pipes.
3. It also influences the working of dyeing.
4. It develops scales in the boilers.
5. It consumes more soap.
Oil from boiler feed water can be removed by the following two methods:
(1) Alum and soda ash are fed into the tank containing water contaminated by oil. The floe produced as a result of reaction of alum and soda ash attract and enmeshes the oil in the floe. This floe is removed by filtering through graded anthrafile bed at regular intervals and the filter is back washed.
The filter is then washed with hot NaOH solution, which dissolves adherent floe, and frees its oil content and finally filter bed is cleaned.
(2) Oil can also be coagulated electrically by cataphoresis in which water containing oil in emulsion is allowed to pass between electrodes of iron or other suitable metal.
Treatment Process # 7. Removal of Slime and Algae from Water:
Hay bacteria (Bacillus subtilis), which are air borne organisms are mainly responsible for slime and algae in water. In industrial plants, slime and alage may cause serious trouble, especially in cooling systems such as air conditioning etc., where spray nozzles and even screens of circulating pumps get clogged by slime and algae.
The latter can be removed from water by screening or by prechlorination. Chemical treatment can also prevent the growth of slime and algae provided the chemical employed is non-toxic, non-volatile, non-odorous and corrodes the metal parts of the plant.
Generally the growth of slime and algae is prevented by:
(a) Using an overdose of chlorine or ozone in water
(b) Using salts of silver, mercury or copper,
(c) Using organic compounds, such as quaternary ammonium compounds and sodiumpenta-chloraphenate.
In order to avoid the development of immunity to a particular chemical in a particular ratio it is desirable to use different chemicals and various doses at different times.
Treatment Process # 8. Removal of Radio-activity from Water:
Radio-active materials may pollute the sources of water supply which is used for drinking purposes. Dangerous materials may be mixed with water due to molecular blasts, wastes from atomic energy installations or use in research, industry or medicine. The wastes from atomic energy installations are so controlled that appreciable health hazards unlikely.
Radioactive substances from water can be removed by distillation, settling, filtration, coagulation, adsorption on sand/day/activated carbon/metals or on other absorbents, by ion exchange processes. Remove upto 80 to 90% can be expected.
Treatment Process # 9. Removal of Dissolved Minerals:
Kenzilite and Zepholite proprietary, ion-exchange compounds, have been used successfully in the removal of lead, zinc, copper and tin. According to Mr. Stretcher, water having dissolved solids from 1000 to 3000 ppm may be demineralized successfully by the application of a direct electric current in specially designed cells with canvas or similar diaphragms.
The hard water has to be made soft by certain methods before it is supplied to the consumers. The process of decreasing the concentration of calcium and magnesium salts in water is called softening.
Types of Hardness:
Hardness is of two types:
(1) Temporary harness.
(2) Permanent hardness.
Temporary hardness is caused due to the presence of bicarbonates of calcium and magnesium and is also known as carbonate hardness.
The permanent hardness is caused by the presence of sulphates, chlorides of calcium and magnesium. This is also called non-carbonate hardness.
Softening of Water:
Removal of temporary hardness:
This hardness of water can be removed by boiling as follows.
Ca(HCO3)2 + Heating CaCO3 + H2O + CO2
Removal of permanent hardness:
The following three methods may be adopted for this purpose:
1. Zeolite process
2. Demineralization process and
3. Lime soda process.
1. Lime soda process:
The process is used for the removal of temporary as well as permanent hardness.
Ca(HCO3)2 + Ca(OH)2 → 2CaCO3 + 2H2O
Mg(HCO3)2 + Ca(OH)2 → CaCO3 + MgCO3 + 2H2O
MgCO3 + Ca(OH)2 → Mg(OH)2 + CaCO3
MgSO4 + Ca(OH)2 → Mg(OH)2 + CaSO4
CaSO4 + Na2CO3 → CaCO3 + Na2SO4
MgCl2 + Ca(OH)2 → Mg(OH)2 + CaCl2
CaCl2 + Na2CO3 → CaCO3 + 2NaCl
MgCl2 + Na2CO3 → MgCO3 + 2NaCl
Mg (HCO3)2 + Ca(OH)2 → CaCO3 + MgCO3 + H2O
(a) Excess lime treatment:
In this method, raw water is over treated with lime in order to completely precipitate magnesium. Soda ash is added to neutralize the excess lime, converting all alkalinity to sodium alkalinity. After filtration if the pH is about 8.0 it, will be good for water with hardness of about 30 ppm.
(b) Recarbonation:
In this process excess lime is added to raw water. Excess lime is then neutralized by the action of CO2.
2. Permutit or Zeolit process:
In this method zeolites also known as green sand are used for water softening. Permutit is artificial zeolite called as hydrate of sodium aluminium orthosilicate. It has general formula Na2O. Al2O3. nSiO2.H2O (where n = 5 to 13 and x = 3 to 4).
3. Ion exchange process:
Substances capable of exchanging ions with the electrolyte solution are called ion exchanger and the process as ion exchange process.
Substances that exchange cations are called cation exchangers and those exchanging anions are called anion exchangers. Cation exchangers dissociate into small mobile ions (cations) capable of exchange (e.g. H+) and high molecular anions while anions exchangers fall into small easily moving anions (e.g. OH–) and a high molecular cations (R+n).
Examples of cation exchangers are:
(a) Sulpho acid and hydroxy phenolic
(b) Sulpho acid and carboxylic
(c) carboxylic and hydroxy phenolic.
Examples onion exchangers are:
(a) quaternary ammonium bases
(b) Tertiary amines,
(c) secondary amines,
(d) primary amines.
When the total hardness is greater than the sum of the carbonates and bicarbonates alkalinity, this is called the non-carbonate hardness.
Sewage treatment:
For sewage treatment we have rotating biological contactor, anaerobic filter and grass plots.
RBC can be operated with raw sewage while the anaerobic filter can function as a composite secondary treatment device in which case the sewage should be free from grit and preferably partly homogenized. The filter can also be used as a secondary treatment device for treating effluent from septic tanks.
Overland grass filtration treatment of filter effluent will be useful adjunct for further ‘polishing’ the effluent from filter or RBC.
Based on the studies so far conducted, the process design criteria have been evolved and are summarized briefly as follows:
RBC for treating raw sewage:
Organic loading rate to disc chamber: 16 to 20 gms BOD5/m2/of disc area/day.
Overall BOD removal efficiency: 90%
Detention time in disc chamber: 1.5 hrs
RPM of disc: 5
Temp. of Sewage: 23 to 32°C
Influent BOD concentration: 200 to 300 mg/1
Detenion time in secondary settling tank: 2.25 hours.
Anaerobic filter:
Media (Gravel, broken stone) size of media: 1.9 cms to 2.5 cm.
Depth of media: 115 cms to 125 cm
Hydraulic Loading rate: 3.4 m3/m2/day
Temp. of sewage: 23°C to 33°C
BOD removal efficiency: 79% to 80% for influent BOD concentration of 110 mg/1 to 300 mg/l.
Detention time in filter: 6 to 12 hours.
Grass Plot: Hydraulic Loading:
0.8 to 1.5 m3/day/sq. meter of area minimum 2 plots.
Slope: 1 in 80 to 120. BOD removal efficiency: 30 to 60 percent.
Chlorinators for disinfection of Drinking Water:
Direct feed type chlorinator:
A simple method of disinfection for piped supplies in to feed bleaching powder solution directly in the suction line of the centrifugal pump, used for drawing water. The bleaching powder is fed from a storage tank through an intermediate container.
The strength of solution used is 1%. The flow into the intermediate container is regulated such that sufficient solution is always present to avoid air bubbles being sucked in. This arrangement is simple and does not require any special gadgets, See fig. 4.
Differential Pressure type Chlorinator:
This type of chlorinator is commercially available for use with piped water supplies. A solution containing bleaching powder and soda ash in proportion of 5: 1 filled in a rubber bag (housed in a metal container) is gradually squeezed out in proportion to the differential pressure across an orifice plate, see fig. 5.
Fig. 4 shows a chlorinator used for application of gaseous chlorine. The figure 4 is self-explanatory to kill the germs in drinking water.
A = Chlorine supply pipe
B = Water supply to chlorine solution.
C = Chloronome of pulsatin meter.
D = Chlorine high pressure gauge.
E = Stop valve.
F = Pressure reducing valve No. 1.
G = Chlorine absorption tower.
H = Chlorine solution outlet pipe connected to main supply.
J = Chlorine low pressure gauge.
K = Regulating valve.
L = No. 2 pressure reducing valve.
M = Connecting tube.
N = Filter
O = Cylinder stop valve.
P = Connector valve.
Q = Cylinder cap.