In this article we will discuss about the methods and techniques used to treat waste water from fertilizer industry. Learn about:- 1. Introduction to Fertiliser Industry 2. Waste-Water from Fertiliser Plants 3. Treatment of Fertiliser Waste-Water 4. Characteristics and Sources of Waste-Water 5. Waste-Water Control Methods 6. Waste-Water Treatment Methods.
Contents:
- Introduction to Fertiliser Industry
- Waste-Water from Fertiliser Plants
- Treatment of Fertiliser Waste-Water
- Characteristics and Sources of Waste-Water from Fertiliser Industry
- Waste-Water Control Methods for Fertiliser Industry
- Waste-Water Treatment Methods for Fertiliser Industry
1. Introduction to Fertiliser Industry:
In Fertiliser plants, a variety of wastes are discharged as water pollutants in the form of processing chemicals like sulphuric acid; process intermediates like ammonia, phosphoric acid; final products like urea, ammonium sulphate, ammonium phosphate, etc. In addition, oil bearing waste from compressor houses of ammonia and urea plants, some portion of the cooling water and the wash water from the scrubbing towers for the purification of gases, also come as waste.
Wash water from, the scrubbing towers may contain toxic substances like arsenic, potassium carbonate, etc. in a nitrogenous fertiliser plant, while that in a phosphatic fertiliser plant may contain a mixture of carbonic acid, hydrofluoric acid and fluorosilicic acid.
Both alkaline and acidic wastes are also expected from the boiler feed water treatment plant, the wastes being generated during the regeneration of anion and cation exchanger units. Fertilisers can be classified as nitrogenous fertilisers, phosphatic fertilisers and complex fertilisers.
Plants may be producing nitrogenous fertilisers, like urea, ammonium sulphate, ammonium nitrate and ammonium chloride, or phosphatic fertilisers like super- phosphates; there are plants where complex fertilisers containing both nitrogen and phosphates, like ammonium phosphate and ammonium sulphate phosphate are produced. Also, some fertiliser units are only involved in mining and undertake no other activity.
2. Waste-Water from Fertiliser Plants
:
A variety of waste streams are discharged from fertiliser plants in the form of:
1. Processing chemicals like sulphuric acid.
2. Process intermediates like ammonia, phosphoric acid, etc.
3. Final products like urea, ammonium sulphate, ammonium phosphate, etc.
In addition, oil bearing wastes from compressor houses of ammonia and urea plants, and some portion of cooling water and wash water from the scrubbing towers for the purification of gases, also come as waste.
Wash water from the scrubbing towers may contain toxic substances like arsenic, monoethanolamine, potassium carbonate, etc. in a nitrogenous fertiliser plant, while that in a phosphatic fertiliser plant may contain a mixture of carbonic acid, hydrofluoric acid and fluosilicic acid.
Both alkaline and acidic wastes are also expected from the boiler feed water treatment plant, the wastes being generated during the regeneration of anion and cation exchanger units. Waste cooling water contained toxic elements like chromates, zinc, etc. which were used for corrosion control.
The development of non-chromate technology using quaternary ammonium compounds has eliminated these toxic substances. Additional pollutants like phenol and cyanide will be introduced in the list of pollutants in a fertiliser plant where ammonia is derived from the waste ammoniacal liquor of coke ovens.
Effects of Wastes on Receiving Streams:
All the components of waste from the fertiliser plants induce adverse effects in streams. Acids and alkalies can destroy normal aquatic life. Arsenic, fluorides, and ammonium salts are found to be toxic to fish. Amines are not only toxic to fish but also exert a high oxygen and chlorine demand.
Presence of different types of salts renders the stream unfit for use as a source of drinking water in the downstream side. Nitrogen and other nutrient content of the waste encourage growth of aquatic plants in the stream.
3. Treatment of Fertiliser Waste-Water
:
Major pollutants in fertiliser waste-water for which treatment is necessary include oil, arsenic, ammonia, urea, phosphate and fluoride. Oil is removed in a gravity separator. Arsenic containing waste is segregated and after its concentration, the solid waste is disposed of in a safe place.
Phosphate and fluoride bearing wastes are also segregated and chemically coagulated by lime; clarified effluent, which still contains some amount of phosphate and fluoride, is diluted by mixing with other wastes.
Several alternatives are there for the treatment of ammonia bearing wastes, including:
1. Steam stripping.
2. Air stripping in towers.
3. Lagooning after pH adjustment.
4. Biological nitrification and denitrification.
For all practical purposes, ‘steam stripping’ for ammonia removal from fertiliser wastes has been found to be uneconomical. Removal of ammonia gas from the solution in an air stripping tower, packed with red wood stakes, is found to be a very efficient method.
Very encouraging results are obtained from some laboratory and pilot plant studies conducted by National Environmental Engineering Research Institute (NEERI) in the removal of ammonia by simply lagooning the waste.
It was found that considerable reduction in the ammonia content can be accomplished just by retaining the ammoniacal waste in an earthen tank, about 1 metre deep, for a day or two, after pretreatment of the waste by lime to increase the pH to 11.0.
However, no reduction in urea content was observed within this period in wastes containing urea; thus waste containing both urea and ammonia required to be retained in the lagoon for a longer period, to allow urea to decompose to ammonia first.
Biological nitrification involves oxidation of ammonia to nitrate, via nitrite under aerobic conditions; this is followed by denitrification of the nitrified effluent under anaerobic condition, in which gaseous N2 and N2O are the end products and are released into the atmosphere.
The denitrification requires addition of some quantity of carbonaceous matter in the reactor. In all the ammonia removal methods, urea remains untouched. If urea removal is required, wastes containing urea must be retained for a sufficiently long time in an earthen lagoon to allow it to decompose first to ammonia.
Pollution control aspects related to single superphosphate (SSP) fertiliser, straight nitrogeneous fertiliser and complex fertilisers are discussed here.
Straight Nitrogenous Fertilisers:
Urea and salts of ammonia are referred to as straight fertilisers and if combined with other nutrients such as phosphates and potash, they are called complex/mixed fertilisers. Nitrogenous fertiliser plants use a large quantity of water mainly for process cooling, steam generation and process use, resulting in waste-water generation at various points in the manufacturing process. In an ammonia plant, if partial oxidation process is used, then the carbon of hydrocarbon feedstock is not completely combusted.
Disposal of the Final Treated Effluent and Monitoring:
Disposal of the final treated effluent to the receiving water system is an important aspect in the pollution control system.
The detailed procedure to be adopted for disposal system is given as under:
1. It is desired that all the effluents after treatment shall be routed to a properly lined guard pond for equalisation and final control.
2. The guard pond should have two compartments, each of at least four hours capacity. All the effluent streams shall be connected to these compartments by a parallel connection system. One compartment of the guard pond shall be used for the routine disposal of effluent, while the other compartment will remain empty and be utilised when effluent do not conform the limits.
3. In the guard pond, an automatic monitoring system for flow and the relevant pollutants shall be provided with high-level alarm system. The parameters necessary for automatic monitoring are pH, ammoniacal nitrogen, nitrate nitrogen and hexavalent chromium.
Monitoring of nitrate nitrogen is applicable to the industries where nitric acid is produced and used for production of fertilisers, and the monitoring of hexavalent chromium is applicable to the industries where chromate-based inhibitors are used for cooling water conditioning.
4. When pollutants in the final effluent exceed the stipulated figures as indicated by the alarm system and the effluent stream responsible cannot be identified, all the effluent streams shall flow the empty compartment of the guard pond. The effluent thus stored should be treated before discharge.
5. The area around the guard pond shall be developed with proper road connection and lighting system so that it can be approached easily at any time.
6. Till the continuous monitoring systems are not installed, the industry shall collect grab samples at a four-hour interval and analyse these for pH and ammoniacal nitrogen.
7. The other parameters as relevant to the industry concerned such as total kjeldahl nitrogen, hexavalent chromium, total chromium cyanide, nitrate nitrogen, vanadium, arsenic, suspended solids and oil and grease should be analysed in the grab sample collected once a day at fixed hours.
8. For effective appraisal of the performance of treatment units, the industry shall monitor the concerned parameters at least once a shift before and after treatment.
Phosphate Fertiliser:
Industrial Operation and Waste-Water:
The phosphate manufacturing and phosphate fertiliser industry is a basic chemical manufacturing industry, in which essentially both the mixing and chemical reactions of raw materials are involved in production.
Also, short- and long-term chemical storage and warehousing, as well as loading/unloading and transportation of chemicals, are involved in the operation. In the case of fertiliser production, only the manufacturing of phosphate fertilisers and mixed and blend fertilisers containing phosphate along with nitrogen and/or potassium is presented here.
Regarding waste-water generation, volumes resulting from the production of phosphorus are several orders of magnitude greater than the waste-waters generated in any of the other product categories. Elemental phosphorus is an important waste-water contaminant common to all segments of the phosphate manufacturing industry, if the phossy water (water containing colloidal phosphorus) is not recycled to the phosphorus production facility for reuse.
The major waste-water source in the defluorination processes is the wet scrubbing of contaminants from the gaseous effluent streams. However, process conditions normally permit the use of recirculated contaminated water for this service, thereby effectively reducing the discharged waste-water volume.
4. Characteristics and Sources of Waste-Water from Fertiliser Industry:
Waste-waters from the manufacturing, processing and formulation of inorganic chemicals such as phosphorus compounds, phosphates and phosphate fertilisers cannot be exactly characterised.
The wastewater streams are usually expected to contain trace or large concentrations of all raw materials used in the plant; all intermediate compounds produced during manufacture; all final products, coproducts and by-products; and the auxiliary or processing chemicals employed.
It is desirable from the viewpoint of economics that these substances not be lost, but some losses and spills appear unavoidable and some intentional dumping does take place during housecleaning, vessel emptying and preparation operations.
Few fertiliser plants discharge waste-waters to municipal treatment systems. Most use ponds for the collection and storage of waste-waters, pH control, chemical treatment and settling of suspended solids.
Whenever available retention pond capacities in the phosphate fertiliser industry are exceeded, the waste-water overflows are treated and discharged to nearby surface water bodies. The range of wastewater characteristics and concentrations for typical retention ponds used by the phosphate fertiliser industry.
The specific types of waste-water sources in the phosphate fertiliser industry are:
(i) Water treatment plant wastes from raw water filtration, clarification, softening and deionisation, which principally consist of only the impurities removed from the raw water (such as carbonates, hydroxides, bicarbonates and silica) plus minor quantities of treatment chemicals,
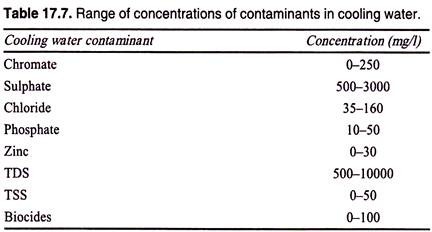
(ii) Closed-loop cooling tower blowdown, the quality of which varies with the makeup of water impurities and inhibitor chemicals used (the only cooling water contamination from process liquids is through mechanical leaks in heat exchanger equipment. Table 17.7 highlights the normal range of contaminants that may be found in cooling water blowdown systems),
(iii) Boiler blowdown, which is similar to cooling tower blowdown but the quality differs as given in Table 17.8,
(iv) Contaminated water or gypsum pond water, which is the impounded and reused water that accumulates sizable concentrations of many cations and anions, but mainly fluorine and phosphorus [concentrations of 8500 mg/l F and in excess of 5000 mg/l P are not unusual; concentrations of radium 226 in recycled gypsum pond water are 60 to 100 picocuries/l and its acidity reaches extremely high levels (pH 1-2)],
(v) Waste-water from spills and leaks that, when possible, is reintroduced directly to the process or into the contaminated water system; and
(vi) Nonpoint source discharges that originate from the dry fertiliser dust covering the general plant area and then dissolving in rain water and snowmelt that become contaminated.
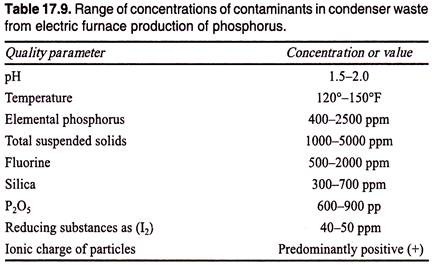
In the specific case of waste-water generated from the condenser water bleedoff in the production of elemental phosphorus from phosphate rock in an electric furnace, Horton reported that the flow varies from 10 to 100 gpm (2.3-23 m3/hr), depending on the particular installation.
The most important contaminants in this waste are elemental phosphorus, which is colloidally dispersed and may ignite if allowed to dry out and fluorine that is also present in the furnace gases. The general characteristics of this type of waste-water (if no soda ash or ammonia were added to the condenser water) are given in Table 17.9.
Fertiliser manufacturing may create problems within all environmental media, i.e. air pollution, water pollution and solid wastes disposal difficulties.
In particular, the liquid waste effluents generated from phosphate and mixed and blend fertiliser production streams originate from a variety of sources and may be summarised as follows:
(i) Ammonia-bearing wastes from ammonia production,
(ii) Ammonium salts such as ammonium phosphate,
(iii) Phosphates and fluoride wastes from phosphate and superphosphate production,
(iv) Acidic spillages from sulphuric acid and phosphoric acid production,
(v) Spent solutions from the regeneration of ion-exchange units,
(vi) Phosphate, chromate, copper sulphate and zinc wastes from cooling tower blowdown,
(vii) Salts of metals such as iron, copper, manganese, molybdenum and cobalt,
(viii) Sludge discharged from clarifiers and backwash water from sand filters, and
(ix) Scrubber wastes from gas purification processes.
Considerable variation, therefore, is observed in quantities and waste-water characteristics at different plants. The most important factors that contribute to excessive in-plant materials losses and therefore, probable subsequent pollution are the age of the facilities (low efficiency, poor process control), the state of maintenance and repair (especially of control equipment), variations in feedstock and difficulties in adjusting processes to cope and an operational management philosophy such as consideration for pollution control and prevention of materials loss.
Because of process cooling requirements, fertiliser manufacturing facilities may have an overall large water demand, with the waste-water effluent discharge largely dependent on the extent of in-plant recirculation. Facilities designed on a once-through process cooling flowstream generally discharge from 1000 to over 10,000 m3/hr waste-water effluents that are primarily cooling water.
According to research results reported by Fuller, the removal of semi-colloidal matter in settling areas or ponds seems to be one of the primary problems concerning water pollution control.
The results of Dissolved Oxygen (DO) and BOD surveys indicated that receiving streams were actually improved in this respect by the effluents from phosphate operations. On the other hand, no detrimental effects on fish were found, but there is the possibility of destruction of fish food aquatic micro-organisms and plankton under certain conditions.
The waste-water characteristics vary from one production facility to the next and even the particular flow magnitude and location of discharge will significantly influence its aquatic environmental impact.
The degree to which a receiving surface water body dilutes a waste-water effluent at the point of discharge is important, as are the minor contaminants that may occasionally have significant impacts.
Fertiliser manufacturing wastes, in general, affect water quality primarily through the contribution of nitrogen and phosphorus, whose impacts have been extensively documented in the literature.
Significant levels of phosphates assist in inducing eutrophication and in many receiving waters they may be more important (growth-limiting agent) than nitrogenous compounds. Under such circumstances, programs to control eutrophication have generally attempted to reduce phosphate concentrations in order to prevent excessive algal and macrophyte growth.
In addition to the above major contaminants, pollution from the discharge of fertiliser manufacturing wastes may be caused by such secondary pollutants as oil and grease, hexavalent chromium, arsenic and fluoride.
As reported by Beg in certain cases, the presence of one or more of these pollutants may have adverse impacts on the quality of a receiving water, due primarily to toxic properties or can be inhibitory to the nitrification process. Finally, oil and grease concentrations may have a significant detrimental effect on the oxygen transfer characteristics of the receiving surface water body.
5. Waste-Water Control Methods
for Fertiliser Industry:
The pollution control and treatment methods and unit processes used are discussed below:
1. In-Plant Control, Recycle and Process Modification:
The primary consideration for in-plant control of pollutants that enter waste streams through random accidental occurrences, such as leaks, spills and process upsets, is establishing loss prevention and recovery systems.
In the case of fertiliser manufacture, a significant portion of contaminants may be separated at the source from process wastes by dedicated recovery systems, improved plant operations, retention of spilled liquids and the installation of localised interceptors of leaks such as oil drip trays for pumps and compressors.
Also, certain treatment systems installed (i.e. ion-exchange, oil recovery and hydrolyser-stripper systems) may, in effect, be recovery systems for direct or indirect reuse of effluent constituents. Finally, the use of effluent gas scrubbers to improve in-plant operations by preventing gaseous product losses may also prevent the airborne deposition of various pollutants within the general plant area, from where they end up as surface drainage runoff contaminants.
2. Cooling Water:
Cooling water constitutes a major portion of the total in-plant wastes in fertiliser manufacturing and it includes water coming into direct contact with the gases processed (largest percentage) and water that has no such contact.
The latter stream can be readily used in a closed-cycle system, but sometimes the direct contact cooling water is also recycled (after treatment to remove dissolved gases and other contaminants and clarification). By recycling, the amount of these waste-waters can be reduced by 80 to 90 per cent, with a corresponding reduction in gas content and suspended solids in the wastes discharged to sewers or surface water.
3. Process Modifications:
The following are possible process modifications and plant arrangements that could help reduce wastewater volumes, contaminant quantities and treatment costs:
(i) In ammonium phosphate production and mixed and blend fertiliser manufacturing, one possibility is the integration of an ammonia process condensate steam stripping column into the condensate boiler feedwater systems of an ammonia plant, with or without stripper bottoms treatment depending on the boiler quality makeup needed,
(ii) Contaminated waste-water collection systems designed so that common contaminant streams can be segregated and treated in minor quantities for improved efficiencies and reduced treatment costs,
(iii) In ammonium phosphate and mixed and blend fertiliser production, another possibility is to design for a lower-pressure steam level (i.e. 42-62 atm) in the ammonia plant to make process condensate recovery easier and less costly; and
(iv) When possible, the installation of air-cooled vapour condensers and heat exchangers would minimise cooling water circulation and subsequent blowdown.
Recently new techniques have been adopted by French company for pollution prevention, for a new process modification for steam segregation and recycle in phosphoric acid production in which, raw water from the sludge/fluorine separation system is recycled to the heat-exchange system of the sulphuric acid dilution unit and the waste-water used in plaster manufacture.
Furthermore, decanted supernatant from the phosphogypsum deposit pond is recycled for treatment in the water filtration unit. This process modification permits an important reduction in pollution by fluorine and that it makes the treatment of effluents easier and in some cases allows specific recycling.
Finally, the new process produced a small reduction in water consumption, either by recycle or discharging a small volume of polluted process water downstream and required no particular equipment and very few alterations in the mainstream lines of the old process.
6. Waste-Water Treatment Methods
for Fertiliser Industry:
Phosphate Manufacturing:
Nemerow summarised the major characteristics of wastes from phosphate and phosphorus compounds production (i.e. clays, limes and tall oils, low pH, high suspended solids, phosphorus, silica and fluoride) and suggested the major treatment and disposal methods such as lagooning, mechanical clarification, coagulation and settling of refined waste-waters.
Phosphate Fertiliser Production:
Contaminated water from the phosphate fertiliser is collected in gypsum ponds and treated for pH adjustment and control of phosphorus and fluorides. Treatment is achieved by ‘double liming’ or a two- stage neutralisation procedure, in which phosphates and fluorides precipitate.
The first treatment stage provides sufficient neutralisation to raise the pH from 1 to 2 to a pH level of at least 8. The resultant effectiveness of the treatment depends on the point of mixing of lime addition and on the constancy of pH control. Fluosilisic acid reacts with lime and precipitates calcium fluoride in this step of the treatment.
The waste-water is again treated with a second lime addition to raise the pH level from 8 to at least 9 (where phosphate removal rates of 95 per cent may be achieved), although two-stage dosing to pH 11 may be employed.
Concentrations of phosphorus and fluoride with a magnitude of 6500 and 9000 mg/l, respectively, can be reduced to 5 to 500 mg/l P and 30 to 60 mg/l F. Soluble orthophosphate and lime react to form an insoluble precipitate, calcium hydroxy apatite.
Sludges formed by lime addition to phosphate wastes from phosphate manufacturing or fertiliser production are generally compact and possess good settling and dewatering characteristics and removal rates of 80 to 90 per cent for both phosphate and fluoride may be readily achieved.
The seepage collection of contaminated water from phosphogypsum ponds and reimpoundment is accomplished by the construction of a seepage collection ditch around the perimeter of the diked storage area and the erection of a secondary dike surrounding the first.
The base of these dikes is usually natural soil from the immediate area and these combined earth/gypsum dikes tend to have continuous seepage through them. The seepage collection ditch between the two dikes needs to be of sufficient depth and size to not only collect contaminated water seepage, but also to permit collection of seeping surface runoff from the immediate outer perimeter of the seepage ditch. This is accomplished by the erection of the small secondary dike, which also serves as a backup or reserve dike in the event of a failure of the primary major dike.
The sulphuric acid plant has boiler blowdown and cooling tower blowdown waste streams, which are uncontaminated. However, accidental spills of acid can and do occur and when they do, the spills contaminate the blowdown streams.
Therefore, neutralisation facilities should be supplied for the blowdown waste streams, which involves the installation of a reliable pH or conductivity continuous- monitoring unit on the plant effluent stream. The second part of the system is a retaining area through which noncontaminated effluent normally flows.
The detection and alarm system, when activated, causes a plant shutdown that allows location of the failure and initiation of necessary repairs. Such a system, therefore, provides the continuous protection of natural drainage waters, as well as the means to correct a process disruption.
Mixed fertiliser treatment technology consists of a closed loop contaminated water system, which includes a retention pond to settle suspended solids. The water is then recycled back to the system.
There are no liquid waste streams associated with the blend fertiliser process, except when liquid air scrubbers are used to prevent air pollution. Dry removals of air pollutants prevent a wastewater stream from being formed.
Phosphate and Fluoride Removal
Phosphates may be removed from waste-waters by the use of chemical precipitation as insoluble calcium phosphate, aluminium phosphate and iron phosphate. The liming process, lime being typically added as a slurry and the system used is designed as either a single or two-stage one.
Polyelectrolytes have been employed in some plants to improve overall settling and clariflocculators or sludge-blanket clarifiers are used in a number of facilities. Alternatively, the dissolved air flotation process is also feasible for phosphate and fluoride removal.
A number of aluminium compounds, such as alum and sodium aluminate, have also been used as phosphate precipitants at an optimum pH range of 5.5 to 6.5, as have iron compounds such as ferrous sulphate, ferric sulphate, ferric chloride and spent pickle liquor.
The optimum pH range for the ferric salts is 4.5 to 5 and for the ferrous salts it is 7 to 8, although both aluminium and iron salts have a tendency to form hydroxyl and phosphate complexes. As reported by Ghokas, sludge solids produced by aluminium and iron salts precipitation of phosphates are generally less settleable and more voluminous than those produced by lime treatment.
According to Sprecht, in the two-step process to remove fluorides and phosphoric acid, water entering the first step may contain about 1700 mg/l F and 5000 mg/l P2O5 and it is treated with lime slurry or ground limestone to a pH of 3.2 to 3.8.
Insoluble calcium fluorides settle out and the fluoride concentration is lowered to about 50 mg/l F, whereas the P2O5 content is reduced only slightly. The clarified supernatant is transferred to another collection area where lime slurry is added to bring the solution to pH 7 and the resultant precipitate of P is removed by settling.
The final clear water, which contains only 3 to 5 mg/l F and practically no P2O5, is either returned to the plant for reuse or discharged to surface waters. The two- step process is required to reduce fluorides in the water below 25 mg/l F, because a single-step treatment to pH 7 lowers the fluoride content only to 25 to 40 mg/l F.
In the process where the triple phosphate is to be granulated or nodulised, the material is transferred directly from the reaction mixer to a rotary dryer and the fluorides in the dryer gases are scrubbed with water. In making defluorinated phosphate by heating phosphate rock, one method of fluoride recovery consists of absorption in a tower of lump limestone at temperatures above the dewpoint of the stack gas, where the reaction product separates from the limestone lumps in the form of fines.
A second method of recovery consists of passing the gases through a series of water sprays in three separate spray chambers, of which the first one is used primarily as a cooling chamber for the hot exit gases of the furnace. In the second chamber, the acidic water is recycled to bring its concentration to about 5 per cent equivalence of hydrofluoric acid in the effluent, by withdrawing acid and adding freshwater to the system.
In the final chamber, scrubbing is supplemented by adding finely ground limestone blown into the chamber with the entering gases. Hydrochloric acid is sometimes formed as a by-product from the fluoride recovery in the spray chambers and this is neutralised with NaOH and lime slurry before being transferred to settling areas.